The NH3 concentration in the receiving water is calculated from the NH4 concentration. Ammonia nitrogen can exist in two states in natural waters: ammonium ion (NH4+) and un-ionized ammonia (NH3). The fraction of total ammonia in un-ionized form is a function of pH and temperature, Emerson (1975):
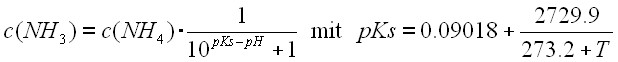
The higher temperature and pH the higher the fraction of un-ionized ammonia in the water. Therefore mostly aquatic life in small, unshaded water courses are vulnerable.
At each time step where a discharge from combined or separate sewer system occurs the NH3 dose (=c(NH3)*Δt) is added and compared to the critical dose. If the actual dose exceeds the critical dose the event is considered to be critical. These critical events are added over the simulation time. At the end the number of critical events per year is computed and displayed.
Keep in mind that only critical events can occur when discharges from combined or separate sewer system take place. A discharge from the separate sewer system must be greater than 10% of the baseflow to take into account. An initial level of NH4 pollution for the receiving water alone does not lead to a critical event. The initial level only enhances the probability that the critical dose is exceeded when a discharge occurs. This is intended and not a program error because the aim of the program is to assess impairments by intermittent discharges of urban drainage during rainfall and not to assess impacts due to chronic non-point pollution.